Our team at GMU has successfully applied our particle-based enrichment and discovery process in the analysis of plasma from multiple reptilian species including the American alligator and Komodo dragon. The successes that we have realized to date validates our central hypothesis that the host-defense peptidomes of living organisms, extreme species in particular, can provide a rich resource for the development of new antimicrobials and it demonstrates the power of the discovery process that we have developed to mine these peptidomes.
- We have invented a novel robust process for the discovery and identification of low abundance antimicrobial host-defense peptides in their natural forms from biological samples. It is capable of identifying peptides and proteins that are virtually undetectable using conventional proteomic methods. We have successfully employed this process to identify novel CAMPs from American alligator, Komodo dragon, and other reptiles. Moreover, we have successfully applied this process to identify differences in the host-defense peptide profiles in plasma from healthy humans and individuals suffering from sepsis.
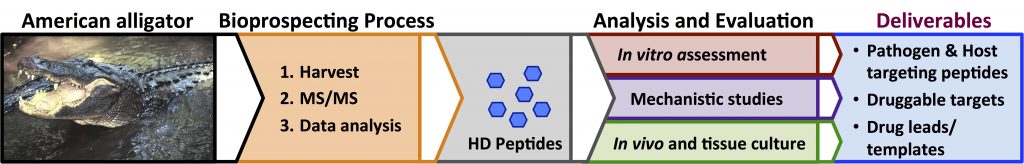
- We have identified multiple novel peptides from the American alligator that show potent antimicrobial properties. Of particular interest are a pair of peptides, Apo5 and Apo6, which were derived from Apolipoprotein C-1. These peptides show broad-spectrum antimicrobial properties and are effective against multiple antibiotic resistant strains of bacteria.
- We have designed a peptide, DRGN-1, based on the sequence of a peptide identified from Komodo dragon plasma that demonstrates potent wound healing properties through a combination of pathogen and host directed effects. It exerts antimicrobial activity against and disrupts biofilm formation by bacteria and at the same time stimulates host cells to promote wound healing. Thereby exerting both pathogen and host directed beneficial effects.
- We have identified a host of novel peptides from Komodo dragon plasma that exhibit broad-spectrum antimicrobial properties, including anthrax and multidrug-resistant bacteria. These peptides would be virtually undetectable using conventional CAMP discovery methods.
- By studying the mechanisms by which these peptides exert their antimicrobial effects, we have identified previously unknown bacterial targets, such as cytoplasmic acyl carrier protein (AcpP). Bacterial AcpP is sufficiently different and is represented in multiple bacterial strains that it represents a new potential target for the development of new classes of broad-spectrum antibiotics.
